
Published Articles

Abstract
Keto–enol tautomerism plays a very important role in pyruvate chemistry and metabolic transformations. The enol form of pyruvate is thermodynamically much less stable, and its tautomerization to the keto form is one of the strongly favored energetic single rearrangements, endowing phosphoenolpyruvate as one of the most potent phosphate donors in biology. In this work, we report the keto–enol rearrangement of pyruvate and pyruvate-derived compounds, such as zymomate, using high-quality density functional theory for the gas phase and molecular dynamics methods for the aqueous phase. Our results indicate a preference for the keto tautomer of pyruvic acid and pyruvate both in the gas as well as in the aqueous phase. Zymonic acid prefers to be in the enol form in gas as well as in an aqueous medium. Tautomerization contributes approximately 42% of the energetic driving force in the hydrolysis of phosphenol pyruvate. Keto tautomer of zymonate, however, is lower in energy in the gas phase, while the enol tautomer, due to its strong solvation free energy, is lower in energy in the aqueous phase. The results of our work are also discussed in the context of astrobiology, where we explore how fundamental carbonyl chemistry such as keto–enol tautomerization reactions may have influenced the selection of phosphoenolpyruvate. We propose a scenario for how the emergence of PEP might have taken place through a process of chemical evolution originating from a wider variety of possible enol phosphates operating within a proto-metabolism.
Published July 17, 2025

Published July 17, 2025
Abstract
Astrobiology and the search for evidence of life beyond Earth are now key drivers for planetary science and astronomy missions. Efforts are underway to establish evaluative frameworks to interpret potential signs of life in returned data. However, there is a need for a “before-the-fact” system to assess mission science risk and the potential false negative and false positive results. The Life Detection Knowledge Base (LDKB) is a community-owned web tool that organizes the scientific literature and enables discourse and evaluation of potential biosignatures (defined to the same level of granularity) relative to a set of standard criteria. This article details the development of draft criteria and their utilization as an organizing basis for the LDKB and their vetting by the astrobiology community via two workshops. We report the incorporation of community feedback to generate a finalized set of criteria, which delineate contributing factors to the potential for false negative or false positive results in the search for evidence of life within and beyond our solar system.

Published July 17, 2025
Abstract
The Life Detection Knowledge Base (LDKB) is a community webtool developed to test and evaluate strategies to search for evidence of life beyond Earth, with an emphasis on recognizing potential false-positive and false-negative results. As part of the LDKB framework, we developed a taxonomy of potential biosignatures. The taxonomy brings together a broad array of life-detection strategies into a common and systematic structure that allows for equitable evaluations based on a specific set of criteria, chosen to assess the likelihood of false-positive and false-negative interpretations. The taxonomy is also a tool to organize life-detection strategies in a way that streamlines their infusion into robotic spaceflight missions. This article describes the structure of the taxonomy and its functional qualities. Two accompanying articles detail the overall LDKB framework and the set of criteria used to evaluate potential biosignatures.

Abstract
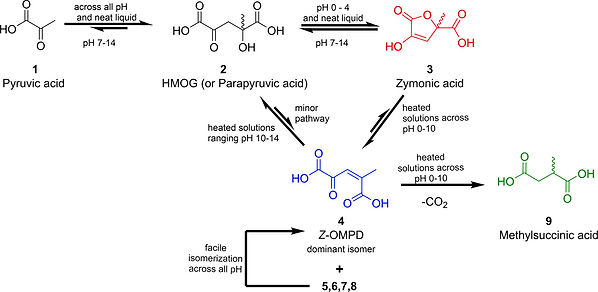
The homoaldol condensation product of pyruvate, 2-methyl-4-oxopent-2-enedioic acid (OMPD), has been recently implicated as a catabolic intermediate in the bacterial degradation of lignin and previously identified from other biological sources in reports ranging over 60 years. Yet, while a preparation of the pyruvate homoaldol product precursor, 4-hydroxy-4-methyl-2-oxoglutaric acid (HMOG/Parapyruvate), was first reported in 1901, there has not been a complete published synthesis of OMPD. Analyses of reaction mixtures have helped identify zymonic acid, the lactone of HMOG, as the direct precursor to OMPD. The reaction appears to proceed through an acid- or base-mediated ring opening that does not involve formal lactone hydrolysis. In addition to a preparative protocol, we provide a proposed mechanism for the formation of methylsuccinic acid that arises from the nonoxidative decarboxylation of OMPD. Finally, we calculated the relative stability of the isomers of OMPD and found Z-OMPD to be the lowest in energy. These computations also support our observations that Z-OMPD is the most abundant isomer across a range of pH values.

Abstract
Carbonaceous meteorites provide the best glimpse into the solar system’s earliest physical and chemical processes. These ancient objects, ~4.56 billion years old, contain evidence of phenomena ranging from solar system formation to the synthesis of organic compounds by aqueous and (likely) low-temperature photolytic reactions. Collectively, chemical reactions resulted in an insoluble kerogen-like carbon phase and a complex mixture of discrete soluble compounds including amino acids, nucleobases, and monosaccharide (or “sugar”) derivatives. This review presents the documented search for sugars and their derivatives in carbonaceous meteorites. We examine early papers, published in the early 1960s, and note the analytical methods used for meteorite analysis as well as conclusions on the results. We then present the recent finding of sugar derivatives including sugar alcohols and several sugar acids: The latter compounds were found to possess unusual “d” enantiomeric (mirror-image) excesses. After discussions on the possible roles of interstellar grain chemistry and meteorite parent body aqueous activity in the synthesis of sugar derivatives, we present a scenario that suggests that most of Earth’s extraterrestrial sugar alcohols (e.g., glycerol) were synthesized by interstellar irradiation and/or cold grain chemistry and that the early solar disk was the location of the initial enantiomeric excesses in meteoritic sugar derivatives.

Published May 31, 2016
Abstract
Biological polymers such as nucleic acids and proteins are constructed of only one—the d or l—of the two possible nonsuperimposable mirror images (enantiomers) of selected organic compounds. However, before the advent of life, it is generally assumed that chemical reactions produced 50:50 (racemic) mixtures of enantiomers, as evidenced by common abiotic laboratory syntheses. Carbonaceous meteorites contain clues to prebiotic chemistry because they preserve a record of some of the Solar System’s earliest (∼4.5 Gy) chemical and physical processes. In multiple carbonaceous meteorites, we show that both rare and common sugar monoacids (aldonic acids) contain significant excesses of the d enantiomer, whereas other (comparable) sugar acids and sugar alcohols are racemic. Although the proposed origins of such excesses are still tentative, the findings imply that meteoritic compounds and/or the processes that operated on meteoritic precursors may have played an ancient role in the enantiomer composition of life’s carbohydrate-related biopolymers.
Published December 8, 2019
Abstract
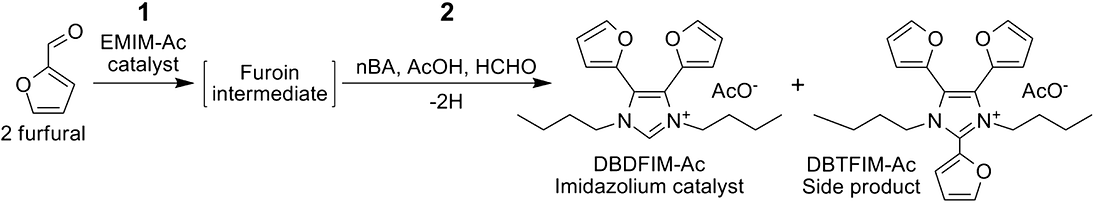
The chemistry of imidazolium-catalyzed imidazolium synthesis was studied as part of an effort to develop a plausible prebiotic synthesis of a small catalytic molecule capable of catalyzing its own synthesis. Specifically, we investigated the one-pot 1-ethyl-3-methylimidazolium acetate (EMIM-Ac) catalyzed synthesis of 1,3-dibutyl-4,5-difuryl-imidazolium acetate (DBDFIM-Ac) from furfural, n-butylamine, formaldehyde, and acetic acid at 80 °C. Liu et al. (2012) had previously demonstrated the first reaction of the synthetic process, the EMIM-Ac catalyzed benzoin condensation of furfural that yields furoin. Our early studies established the second reaction of the synthetic process, the multicomponent reaction of furoin, n-butylamine, formaldehyde, and acetic acid that yields the imidazolium salt, DBDFIM-Ac. Studies of the complete two-reaction process that uses furfural for the synthesis of DBDFIM-Ac showed that the highest yield of DBDFIM-Ac was obtained when the mole ratio of n-butylamine, formaldehyde, and acetic acid relative to furfural was respectively (0.5:0.25:0.25:1.0-furfural), or one-half of the stoichiometric ratio (1.0:0.5:0.5:1.0-furfural). ...

Abstract
In this white paper we discuss the need for system-level analyses of organic molecules present in extraterrestrial samples and the importance of laboratory studies in support of these analyses.

Published January 7, 2015
Abstract
Any discussion related to how life began on this planet inevitably invokes the question as to the origin of bio-organic molecules, a field called prebiotic chemistry (1). How did organic compounds come to populate the early Earth? Before 1953, this question itself was not widely considered within the realm of
experimental science. However, since the pioneering results of the Miller–Urey experiment that produced amino acids from electrical discharges passing through simple gases (2), the field of prebiotic chemistry has been extremely prodigious in demonstrating abiotic syntheses for multitudes of organic compounds. However, it became apparent that prebiotic chemistry was faced with a more challenging question. How did the biomolecules of life get selected out of such
complex, prebiotic mixtures?

Abstract
The native bases of RNA and DNA are prominent examples of the narrow selection of organic molecules upon which life is based. How did nature “decide” upon these specific heterocycles? Evidence suggests that many types of heterocycles could have been present on the early Earth. It is therefore likely that the contemporary composition of nucleobases is a result of multiple selection pressures that operated during early chemical and biological evolution. The persistence of the fittest heterocycles in the prebiotic environment towards, for example, hydrolytic and photochemical assaults, may have given some nucleobases a selective advantage for incorporation into the first informational polymers. The prebiotic formation of polymeric nucleic acids employing the native bases remains, however, a challenging problem to reconcile. Hypotheses have proposed that the emerging RNA world may have included many types of nucleobases. This is supported by the extensive utilization of non-canonical nucleobases in extant RNA and the resemblance of many of the modified bases to heterocycles generated in simulated prebiotic chemistry experiments. Selection pressures in the RNA world could have therefore narrowed the composition of the nucleic acid bases. Two such selection pressures may have been related to genetic fidelity and duplex stability. Considering these possible selection criteria, the native bases along with other related heterocycles seem to exhibit a certain level of fitness. We end by discussing the strength of the N-glycosidic bond as a potential fitness parameter in the early DNA world, which may have played a part in the refinement of the alphabetic bases.